radiation
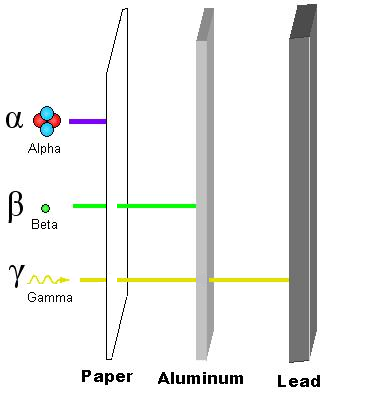
Particle Radiation
Radiation as used in physics, is energy in the form of waves or moving subatomic particles. Radiation can be classified as ionizing or non-ionizing radiation, depending on its effect on atomic matter. The most common use of the word "radiation" refers to ionizing radiation. Ionizing radiation has enough energy to ionize atoms or molecules while non-ionizing radiation does not. Radioactive material is a physical material that emits ionizing radiation.
Types of Radiation
Electromagnetic radiation: (Energy in the form of electromagnetic waves or photons.)
Non-ionizing
Thermal radiation (heat radiation)
Radio waves
Microwave radiation, as used in microwave ovens
Infrared radiation (IR), produced by heat
Visible light - light that is visible to the naked eye
Ultraviolet radiation (UV) is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than soft X-rays.
Ionizing
X-rays, used in radiography for medical diagnosis
Gamma radiation, usually emitted by radioactive atoms
Particle radiation: (Energy in the form of moving subatomic particles.)
Alpha radiation, composed of the nuclei of helium-4 atoms
Beta radiation, consisting of energetic electrons or positrons
Neutron radiation, consisting of neutrons
The effect of magnetic and electric fields on these particles/rays:
Positively charged alpha particles are deflected by both magnetic and electric fields.
Negatively charged beta particles are also deflected by both types of fields, but in the opposite direction from alpha particles.
Neutrons and electromagnetic radiation have no charge, and are unaffected by electromagnetic fields.
lured into a deep sleep at Monday, October 15, 2007 0 person(s) commented while I sleep
